Properties of covalent bonds pogil – Embark on an in-depth exploration of the fundamental properties of covalent bonds, unveiling their formation, polarity, hybridization, and resonance. Delve into the intricate world of molecular interactions and discover the key principles that govern the behavior of covalent bonds.
Covalent bonds, the cornerstone of molecular architecture, arise from the sharing of electron pairs between atoms. This shared ownership bestows unique properties upon molecules, shaping their reactivity, stability, and overall behavior.
Properties of Covalent Bonds
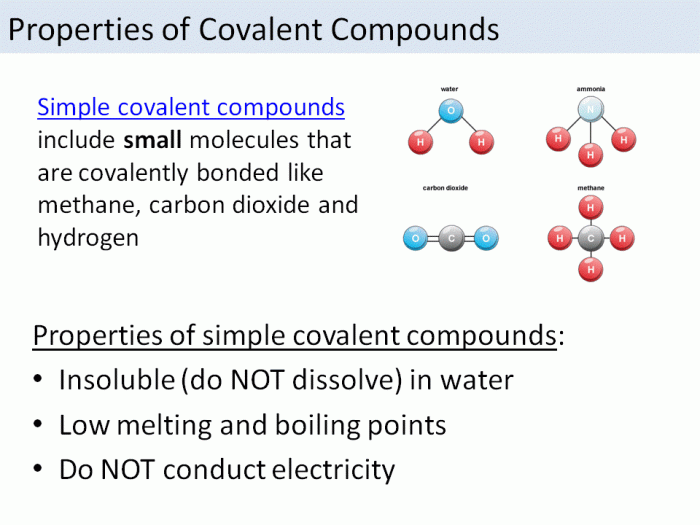
Covalent bonds are formed when two atoms share one or more pairs of electrons. The strength of a covalent bond is determined by the number of electron pairs shared and the distance between the atoms. Covalent bonds are typically stronger than ionic bonds, but weaker than metallic bonds.
Examples of Molecules Formed by Covalent Bonds
- Water (H 2O)
- Methane (CH 4)
- Ammonia (NH 3)
Polarity of Covalent Bonds

Bond polarity refers to the uneven distribution of electrons in a covalent bond. The electronegativity of an atom is a measure of its ability to attract electrons. If two atoms in a covalent bond have different electronegativities, the electrons will be pulled towards the more electronegative atom, creating a polar bond.
Examples of Polar and Nonpolar Covalent Bonds
- Polar covalent bond: Hydrogen chloride (HCl)
- Nonpolar covalent bond: Methane (CH 4)
Hybridization of Covalent Bonds
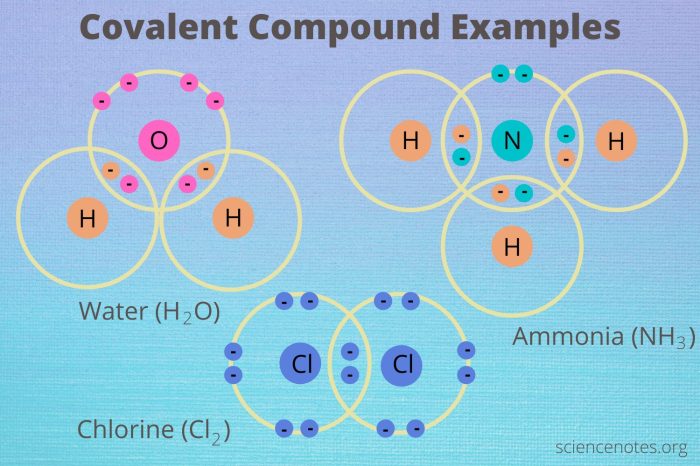
Hybridization is the process of mixing atomic orbitals to form new hybrid orbitals that have different shapes and energies. The type of hybridization that occurs depends on the number and type of atomic orbitals involved.
Different Types of Hybridization and Their Effects on Geometry, Properties of covalent bonds pogil
| Hybridization | Geometry | Examples |
|---|---|---|
| sp | Linear | BeCl2 |
| sp2 | Trigonal planar | BF3 |
| sp3 | Tetrahedral | CH4 |
Resonance in Covalent Bonds
Resonance is a phenomenon that occurs when a molecule can be represented by two or more valid Lewis structures. The resonance structures have the same number of electrons and atoms, but the arrangement of the electrons is different.
Examples of Molecules that Exhibit Resonance
- Benzene (C 6H 6)
- Carbon dioxide (CO 2)
- Ozone (O 3)
FAQ Resource: Properties Of Covalent Bonds Pogil
What is the difference between polar and nonpolar covalent bonds?
Polar covalent bonds arise when the electronegativity difference between the bonded atoms is significant, resulting in an uneven distribution of electrons. Nonpolar covalent bonds, on the other hand, occur when the electronegativity difference is negligible, leading to an equal sharing of electrons.
How does hybridization affect the geometry of molecules?
Hybridization involves the mixing of atomic orbitals to form new hybrid orbitals with specific shapes and orientations. These hybrid orbitals dictate the geometry of molecules, determining their bond angles and molecular shapes.
What is resonance and how does it contribute to molecular stability?
Resonance occurs when a molecule can be represented by multiple Lewis structures that differ in the placement of electrons. This delocalization of electrons enhances the stability of the molecule by lowering its overall energy.