Organizing the periodic table worksheet – Embarking on a journey to unravel the intricacies of the periodic table, this worksheet serves as an invaluable tool to guide students through the systematic organization of elements. Delving into the fundamental principles that govern the periodic table’s structure, this worksheet empowers learners to grasp the properties and characteristics that dictate an element’s position within this enigmatic table.
Venturing further, the worksheet explores the vertical columns (groups) and horizontal rows (periods) that define the periodic table’s layout. By examining representative elements within each group and period, students will uncover the commonalities and differences that shape their chemical identities.
1. Periodic Table Basics
The periodic table is a systematic arrangement of chemical elements based on their atomic number, electron configuration, and recurring chemical properties. It provides a concise overview of the known elements, facilitating the study and understanding of their properties and behaviors.
Properties and Characteristics
The position of an element within the periodic table is determined by its atomic number, which represents the number of protons in its nucleus. Elements with similar chemical properties are grouped together vertically in columns called groups. Horizontally, elements are arranged in rows called periods, indicating the number of electron shells they possess.
2. Groups and Periods: Organizing The Periodic Table Worksheet
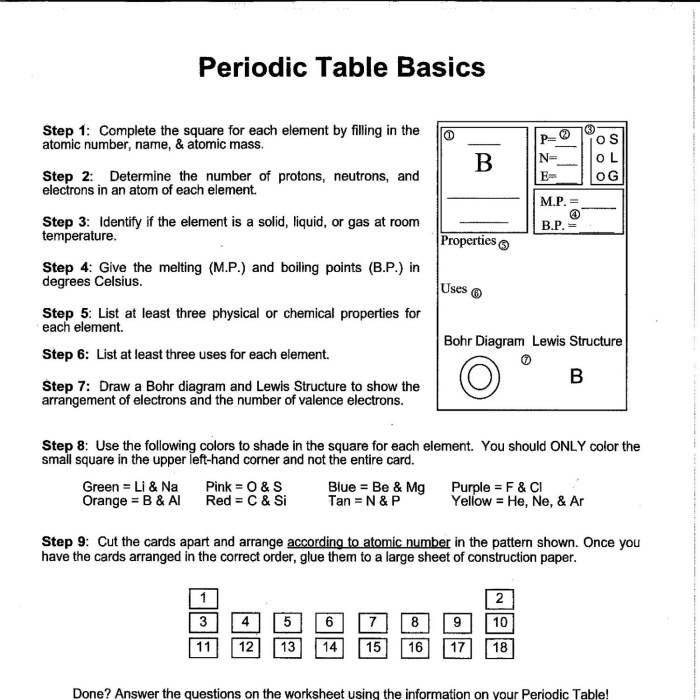
Groups (Vertical Columns)
Groups, also known as families, are vertical columns in the periodic table that contain elements with similar chemical properties. Elements within a group have the same number of valence electrons, which are the electrons in the outermost shell of an atom.
This similarity in valence electrons results in similar chemical reactivity and behavior.
- Group 1: Alkali metals
- Group 2: Alkaline earth metals
- Group 17: Halogens
- Group 18: Noble gases
Periods (Horizontal Rows)
Periods are horizontal rows in the periodic table that represent the number of electron shells in an element’s atoms. Elements within a period have the same number of electron shells but differ in their atomic number and number of valence electrons.
- Period 1: Contains two elements (hydrogen and helium) with one electron shell.
- Period 2: Contains eight elements with two electron shells.
- Period 3: Contains eight elements with three electron shells.
3. Organizing Elements
The organization of elements in the periodic table is based on several criteria, including:
- Atomic number: The number of protons in an atom’s nucleus.
- Electron configuration: The arrangement of electrons in an atom’s electron shells.
- Chemical reactivity: The tendency of an element to react with other elements.
Concept of Periodicity, Organizing the periodic table worksheet
Periodicity refers to the recurring patterns of chemical and physical properties observed when elements are arranged in order of their atomic number. These patterns allow scientists to predict the properties of undiscovered elements and understand the behavior of known elements.
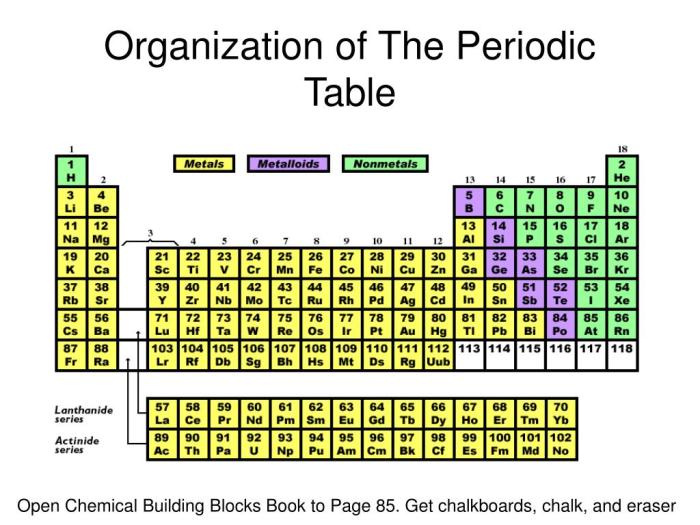
4. Metals, Nonmetals, and Metalloids
Definition and Distinction
Elements can be classified into three main categories based on their properties and location on the periodic table:
- Metals: Located on the left-hand side of the periodic table, metals are generally shiny, malleable, ductile, and good conductors of heat and electricity.
- Nonmetals: Located on the right-hand side of the periodic table, nonmetals are typically dull, brittle, and poor conductors of heat and electricity.
- Metalloids: Located along the diagonal line between metals and nonmetals, metalloids possess properties of both categories.
Examples
- Metals: Sodium (Na), Iron (Fe), Gold (Au)
- Nonmetals: Oxygen (O), Chlorine (Cl), Carbon (C)
- Metalloids: Silicon (Si), Germanium (Ge), Arsenic (As)
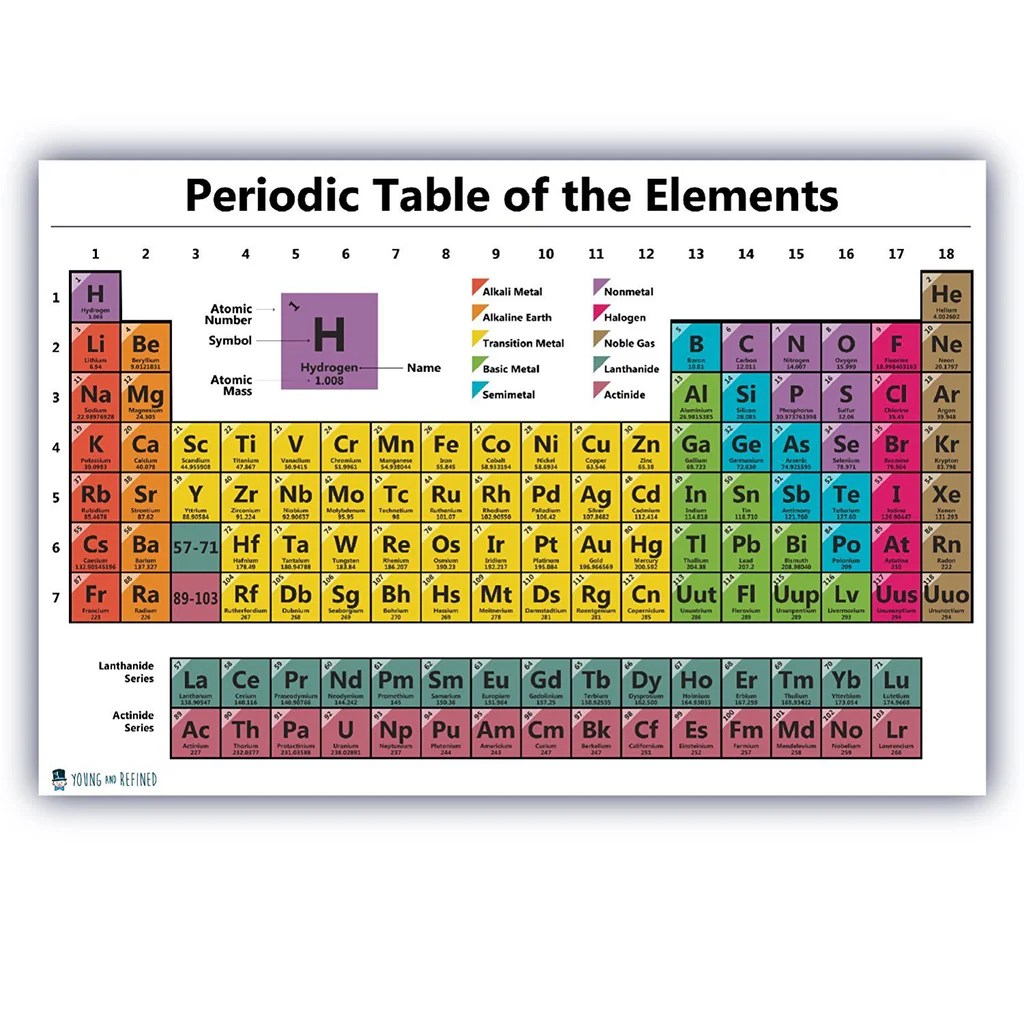
5. Representative and Transition Elements
Identification and Differences
Elements can also be classified into two main groups based on their electron configurations:
- Representative elements (also known as main-group elements): Occupy groups 1-2 and 13-18 in the periodic table. They have their valence electrons in the outermost shell.
- Transition elements: Occupy groups 3-12 in the periodic table. They have valence electrons in both the outermost shell and the penultimate shell (the shell just below the outermost shell).
Properties and Characteristics
- Representative elements: Generally exhibit predictable chemical behavior and are more reactive than transition elements.
- Transition elements: Exhibit a wider range of oxidation states and form complex compounds with varying colors.
6. Organizing Worksheets
Design and Purpose
Worksheets can be designed to guide students through the organization of the periodic table. These worksheets can include sections for:
- Classifying elements into metals, nonmetals, and metalloids
- Identifying trends in atomic number, electron configuration, and chemical reactivity
- Exploring relationships between elements within groups and periods
Clarifying Questions
What are the key principles behind organizing the periodic table?
The periodic table is organized based on atomic number, electron configuration, and chemical reactivity, which determine an element’s properties and position within the table.
How are elements grouped within the periodic table?
Elements are grouped vertically into 18 groups based on their electron configurations and chemical properties. Horizontally, they are arranged into 7 periods based on their energy levels.
What is the significance of periodicity in the periodic table?
Periodicity refers to the repeating patterns of properties observed when moving across or down the periodic table. It allows for the prediction of an element’s properties based on its position within the table.