Section 3 writing formulas and naming compounds provide a fundamental framework for understanding the language of chemistry. These formulas and naming conventions enable chemists to communicate precisely about the composition and structure of chemical substances, facilitating collaboration, research, and technological advancements.
This guide delves into the intricacies of Section 3 writing formulas, exploring their purpose, types, and applications in naming both ionic and covalent compounds. With a comprehensive table of examples and in-depth explanations, this resource empowers learners to confidently navigate the complexities of chemical nomenclature.
Introduction to Section 3 Writing Formulas: Section 3 Writing Formulas And Naming Compounds
Section 3 writing formulas are a set of rules used to represent the chemical composition of compounds. These formulas provide a concise and systematic way to describe the elements and their proportions within a compound.
There are two main types of Section 3 writing formulas: empirical formulas and molecular formulas. Empirical formulas show the simplest whole-number ratio of elements in a compound, while molecular formulas indicate the exact number of atoms of each element in a molecule.
Naming Compounds Using Section 3 Writing Formulas, Section 3 writing formulas and naming compounds
Section 3 writing formulas can be used to name ionic and covalent compounds.
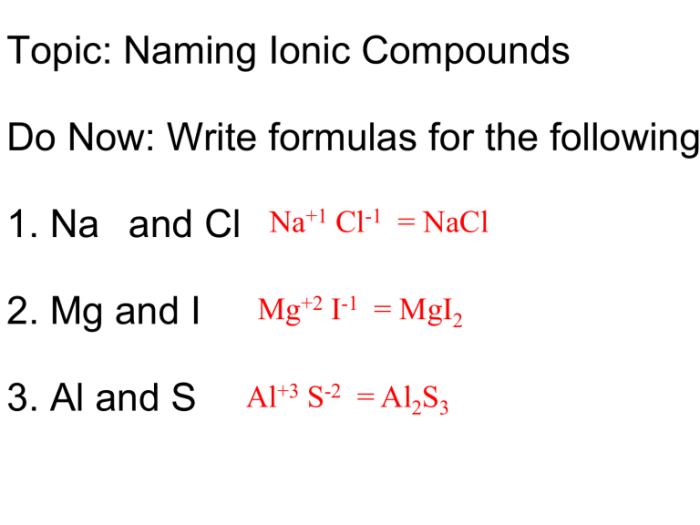
Ionic Compounds
Ionic compounds are formed by the transfer of electrons between metal and non-metal atoms. To name an ionic compound, the name of the metal is followed by the name of the non-metal, with the ending “-ide”. For example, NaCl is sodium chloride.
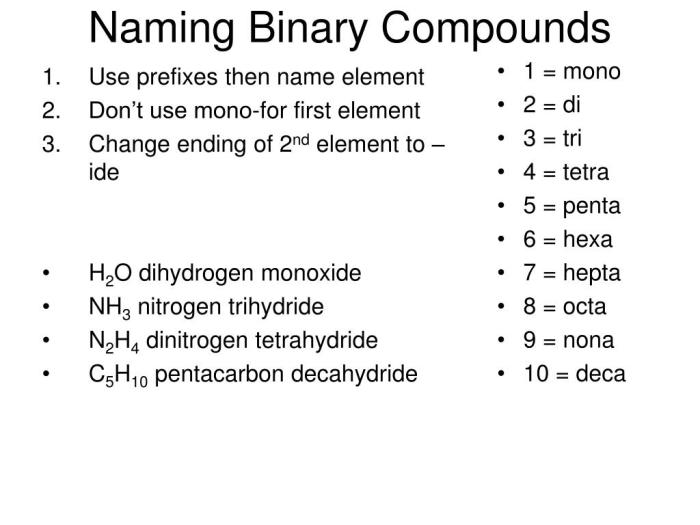
Covalent Compounds
Covalent compounds are formed by the sharing of electrons between atoms. To name a covalent compound, the prefixes “mono-“, “di-“, “tri-“, etc., are used to indicate the number of atoms of each element in the molecule. For example, CO2 is carbon dioxide.
Examples of Section 3 Writing Formulas
| Compound | Formula | IUPAC Name |
|---|---|---|
| Sodium chloride | NaCl | Sodium chloride |
| Carbon dioxide | CO2 | Carbon dioxide |
| Water | H2O | Water |
| Methane | CH4 | Methane |
The formula of a compound can be used to determine its name, and vice versa. For example, the formula NaCl represents sodium chloride, and the name sodium chloride corresponds to the formula NaCl.
Advanced Applications of Section 3 Writing Formulas
Section 3 writing formulas can be used to predict the properties of compounds. For example, the formula of a compound can be used to predict its solubility, acidity, and basicity.
However, it is important to note that Section 3 writing formulas have limitations. For example, they cannot be used to predict the geometry of a compound or the strength of its chemical bonds.
FAQ Explained
What is the purpose of Section 3 writing formulas?
Section 3 writing formulas provide a systematic method for representing the composition and structure of chemical compounds, facilitating communication and understanding among chemists.
How are ionic compounds named using Section 3 writing formulas?
Ionic compounds are named by combining the name of the cation (positive ion) with the name of the anion (negative ion), followed by the appropriate charge.
What are the rules for naming covalent compounds using Section 3 writing formulas?
Covalent compounds are named using prefixes to indicate the number of atoms of each element present in the molecule, followed by the root name of the element and the suffix “-ide”.